Monkeypox Disease
"Unmasking the Fight Against Monkeypox: Challenging the Virus, Empowering the Market Growth"
The non-endemic mpox (Monkeypox) outbreak in the phase of 2022 & 2023 amidst the COVID-19 pandemic is of interest not only due to its impact on medical conditions, but also on economic, and societal climates.
There are several cases of monkeypox that have been reported to WHO from different countries worldwide since January 1, 2022. In the current scenario of the year 2023 a total of 88,144 laboratory-confirmed cases and 1,084 suspected cases, including 149 fatalities, have been reported to WHO which has risen a major health concern to the world.
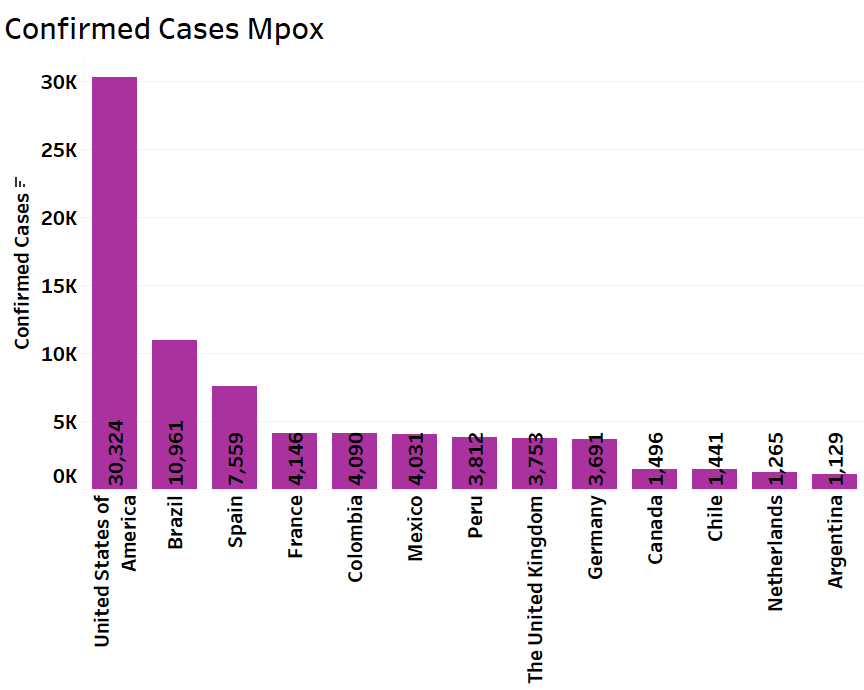
Following is the graphical presentation of the major 20 countries that have significantly reported of total 38,020 confirm monkeypox cases in the year 2022-23. According to WHO, Countries with higher risk include the United States of America, Spain, Brazil, the United Kingdom, and France. As per WHO Committee Members, they have assessed the global risk for monkeypox disease as Moderate.
Representation of Monkeypox Confirmatory Cases Worldwide
The main cause of monkeypox disease transmission is from animals to people. Humans are more susceptible to monkeypox through coming into intimate contact with an animal or person who has the diagnosed with mpox. According to the CDC report on the outbreak of monkeypox states, men having sex with men (MSM) is the other major cause of MPX cases, placing bisexual, transgender, and gay people at an increased risk of catching MPX.
Moreover, it was found that between April-June 2022 that 98% of monkeypox infections in 16 countries across the globe were men who had sex with men and the LGBT community.
Based upon the DLI analysis, as of September 8, 2022, 56,609 cases and 18 deaths had been reported worldwide with the affected nation being United States, Spain, and France. Among the countries, the United Kingdom has the most severe fatality rate.
Currently, to treat Monkeypox, there has been no dedicated drug therapy that can be used. On many occasions, an antiviral drug that was initially developed to treat smallpox is officially used to cure the disease. This brings many opportunities for market players to invest in this sector and gain new business value.
The market for infections with monkeypox is projected to be influenced by factors comprising the increasing incidence of monkeypox infection nationwide. The other factors such as the increased frequency of medical conditions worldwide and the difficulties in determining the underlying causes of illness development, the sector is expanding significantly.
As a result of the monkeypox outbreak, there are higher hospitalizations and thus, increased demand for therapeutic products for symptomatic treatment has become an important factor for market growth. In addition to the above, the government's initiatives from different regions to halt the monkeypox virus's spread and stop the outbreak of a new pandemic are also assisting the industry's development.
Disease Landscape insights facilitate making informed choices in the field of medical technology, treatments, and diagnostics where different technology and remarkable advancements will transform the landscape leading to higher growth.
Diagnostic Analysis
"Diagnostic Innovation for Monkeypox: Empowering Patient Care"
One of the main drivers for the growth of the global monkeypox disease diagnostic market is the growing popularity of confirmatory testing by PCR and easily accessible home kits like Rapid Antigen Test. Below are the main diagnostic categories that contribute significantly to the market depending on various factors such as the advancements in diagnostic technologies, and the prevalence of the disease healthcare infrastructure. It has been discovered that laboratory and molecular tests represent 65% of the diagnostic marketplace for monkeypox. Diagnostic categories are as follows.
- Laboratory Testing.
- Clinical Evaluation.
- Isolation testing
- Serological Testing
Laboratory Testing: Polymerase Chain Reaction (PCR) testing is frequently used to identify the genetic material of the monkeypox virus in patient samples, Monkeypox can be accurately diagnosed using PCR. Due to its wide availability and quick test times, the PCR technology segment generated the biggest portion of the total income.
Clinical Evaluation: Medical professionals evaluate the patient's symptoms, particularly the distinctive rash in monkeypox and related flu-like signs and symptoms. They also consider the patient's past exposure to suspected monkeypox infection sources, such as interaction with animals or infected people. The hospitals and clinics being the end-user segment these segments is expected to grow at the fastest growth rate.
Isolation testing-The existence of the monkeypox virus can be determined by isolating the virus from patient samples and cultivating it in a lab.
Serological Testing: Blood samples can be examined for the existence of certain anti-monkeypox virus antibodies. If a person has had monkeypox before, serological testing like the enzyme-linked immunosorbent assay (ELISA) or neutralization tests can assist detect the monkeypox virus.
Many healthcare agencies and governments are taking steps to increase testing, which is accelerating the market's expansion. For instance, in September 2022, the U.S. FDA as part of its continuous effort to deal with existing outbreaks announced various programs and significant actions to conduct diagnostic tests in all possible rural and urban areas. PCR tests with their remarkable specificity, sensitivity, and speed amplification have led to propel the growth in Monkeypox disease diagnostic market. PCR has challenged the other diagnostic test for confirmation of Monkeypox disease. However, PCR has known to be dominating confirmatory test as it has its accuracy on the other test kits in the market.
Top Players of Diagnostic Kits
The global monkeypox testing market is highly competitive and owing to the number of large market players with a global presence below in the given table there is list of top manufacturers of diagnostic kits for monkeypox globally.
|
Sr No |
Manufacturer |
Product Portfolio |
|
1 |
ACON Biotech |
Promotor Monkeypox Virus Real Time PCR Test Kit |
|
2 |
Altona Diagnostics |
Real Star Zoonotic Orthopoxvirus PCR kit 1.0 |
|
3 |
Bioperfectus Technologies |
Monkeypox Virus Real Time PCR Kit |
|
4 |
DaAn Gene |
Detection Kit for Monkeypox Virus DNA |
|
5 |
hanghai ZJ Bio-Tech Co., Ltd. |
Monkeypox Virus Real Time PCR Kit |
|
6 |
NOVACYT |
GenesigMonkeypox virus M3L gene |
|
7 |
Perkin Elmer |
Pkamp Monkeypox Virus RT-PCR RUO Kit |
|
8 |
Sansure Biotech |
Monkeypox virus Nucleic Acid Diagnostic Kit |
|
9 |
ThermoFisher |
TaqMan Monkeypox Virus Microbe Detection |
|
10 |
TIB Molbiolc |
LightMix Modular Monkeypox Virus |
|
11 |
TRIVITRON |
Vitro detection of Monkeypox and Smallpox virus |
|
12
|
Alpha diagnostic Interenational |
Recombivirus Mon key Anti monkeypox IgG ELISA kit |
|
13 |
Creative diagnostics |
Human Anti-MPV IgM (Anti-Monkeypox Virus IgM) ELISA Kit |
|
14 |
JOYSIBO |
ELISA KIT |
|
15 |
GENES 2 ME |
Real-Time PCR |
|
16 |
Becton, Dickinson, and Company |
molecular polymerase chain reaction (PCR) assay for monkeypox virus |
Treatment Analysis
"Fighting Back: Breakthroughs in Monkeypox Treatment Analysis"
Monkeypox is treated with supportive care. Vaccines and therapeutics developed for smallpox and approved for use in some countries can be used for Monkeypox. Treating the rash, controlling the pain, and avoiding complications are the main objectives of Monkeypox treatment. The smallpox vaccine is given to protect against the monkeypox virus and reduce the severity of monkeypox infection. Highly preferred tecovirimat as their antiviral medication of choice.
The European Medicines Agency (EMA) has approved one of the two antiviral medicines already available for the treatment of smallpox and monkeypox (tecovirimat) for the management of monkeypox. To evaluate the effectiveness of tecovirimat in enhancing clinical outcomes during this monkeypox outbreak, a global collaborative clinical research protocol has been created.
The global market for monkeypox treatments is segmented based on the therapy, end-user competitive environment, and regional distribution. The smallpox vaccination, antivirals, and vaccinia immune globulin (VIG) are the three therapeutic categories that collectively make up the market. Due to the increasing number of cases of monkeypox, the smallpox vaccine category is projected to have the highest share in the global market for monkeypox therapies (preventive).
There are antiviral drugs that can be used to treat monkeypox (curative). Some of these drugs were created to treat animal smallpox. Along with that patients who have severe disease have also benefited from combined therapy of tecovirimat and cidofovir. Three main antiviral drug treatments, including variola (the virus that causes smallpox) in animals and in vitro tests, have shown success against orthopoxviruses.
1) Antivirals’ Drugs Used for Treatment
The below table shows the three antivirals’ drugs used for the treatment of mpox currently with information on the mode of action, typical dosing, formulation, and side effects:
|
Therapy |
Mode of Action |
Typical Dosing |
Formulation |
Side Effects |
|
Cidofovir |
Blocks viral DNA synthesis through competitive inhibition of DNA polymerase |
5 mg/kg per dose once weekly for ≥2 doses (with concomitant probenecid) |
IV; off-label: topical, intravascular |
Nephrotoxicity; neutropenia; decreased intraocular pressure, nausea, vomiting |
|
Tecovirimat |
Inhibits activity of the protein VP37, which prevents creation of virions that can be released from an infected host cell, thereby preventing replication and dissemination within the host |
IV: 35 to <120 kg: 200 mg q12 hours |
IV and oral (off-label topical) |
IV: pain and swelling at infusion site; extravasation at infusion site; headache |
|
Brin cidofovir |
Lipid conjugate prodrug of cidofovir |
4 mg/kg once weekly for 2 doses (max 200 mg/dose) |
Oral |
Abdominal pain, nausea, vomiting, diarrhoea, elevated liver transaminases and bilirubin |
For above mentioned three drugs, FDA has given approval for research in the United States in 18 studies of infected samples totalling 71 subjects. Tecovirimat was used by 61 people, cidofovir by seven people, and Brin cidofovir (BCV) by three people. In addition to tecovirimat, topical trifluridine was administered in four ophthalmic instances. Out of the total, 59 (83.1%) had complete symptom remission; one had waxing and waning symptoms, one (1.8%) had passed away, and the remaining five experienced symptom resolution. The death was assumed not to be connected to tecovirimat. All BCV-treated patients (resulting in treatment cessation) and five tecovirimat-treated patients had elevated liver panels.
The most popular treatment for monkeypox, tecovirimat, has shown promise in several distressing circumstances. Upon use, no significant safety issues for monkeypox drugs were found. Along with tecovirimat, topical trifluridine was utilized as an adjuvant therapy. Infrequently used were BCV and cidofovir, with the latter often utilized because tecovirimat wasn't readily available. BCV was linked to treatment stopping due to the negative side effects of the drug.
"Empowering Immunity: Fighting Against Monkeypox"
2) Vaccine used to prevention from Monkeypox disease.
On August 9, 2022, the FDA just issued JYNNEOS an emergency use authorization (EUA), enabling medical practitioners to administer the vaccine intradermally (between the layers of skin) to adults who are at high risk of contracting monkeypox. Production of vaccine -approximately US$ 240 million of TPOXX has been delivered under this contract. The strategic stockpile obtained US$ 460 million worth of oral TPOXX due to a contract SIGA received by BARDA in 2021. JYNNEOS is given in a two-dose course separated by four weeks. A two-pronged strategy is required for the prevention of monkeypox. According to CDC recommendations, postexposure modified vaccinia Ankara (MVA) immunization is necessary for people who have had high-risk exposure to monkeypox.
Manufacturing of Antiviral Drugs and Vaccine
|
Manufacturer of Anti-Drug & Vaccine |
Product/Brand Name |
|
SIGA Technologies |
Tecovirimat |
|
Chimerix |
Brincidofovir |
|
Emergent |
Tembexa |
|
Bavarian Nordic A/S (Vaccine) |
JYNNEOS (Imvamune or Imvanex) vaccine |
Manufacturers of antiviral medications and monkeypox vaccines will get important insight and knowledge from Disease Landscape Insights. Thus, it helps them with focused research and development to conduct, information on CDMO, CMO, and raw material suppliers, along with legal compliance for the company in production process optimization. Disease Landscape Insights services also help in directing the target population identification, and marketing strategies, and keep market players updated with industry trends. Manufacturers are creating and deploying efficient treatments to stop and manage monkeypox outbreaks by utilizing data from Disease Landscape Insights.
Services Offered by Disease Landscape Insights in Monkeypox Diseases Industry to grow exponentially.
"Collaborative Services for Health and Safety: Working Together for a Society Free from Monkeypox"
Disease Landscape insights serve a purpose in directing and setting up the commercial monkeypox services to leading global players. It supports them by providing market analysis of product launch expansion, research & development in field of drug discovery and launch phase, resource allocation, risk estimation on efficacy and safety of drugs, collaboration services among the supply chain, and long-term planning by providing insights into the dynamics of the disease.
Diseaselandscape Insight provides intensive services in data management to clinical and drug safety concerns, along with that medical writing which involves writing scientific papers for a variety of objectives, like legislative and research-related documents, disease- or associated with drugs instructional and promotional materials, journal articles and abstracts for publications, and website content for the healthcare industry. In delivering regulatory issues, professionals can respond to the expectations of market players within the Healthcare Industry.The commercial aspect of monkeypox disease focuses on a range of services and products, from management and prevention to further treatment and research, which try to address the effects of the illness. Following are the services taken to initiate eliminating the outbreak.
- Five commercial laboratory businesses, including the largest reference laboratory in the country, started receiving orthopoxvirus tests from the Department of Health and Human Services (HHS) through the Centres for Disease Control and Prevention (CDC) since to increase monkeypox testing capacity and have access in every community during the ongoing monkeypox outbreak.
- The businesses are Aegis Science, LabCorp, Mayo Clinic Laboratories, Quest Diagnostics, and Sonic Healthcare.
- The Secretary of Health and Human Services boosted the number of testing centres around the nation, enabling everyone who needs to be tested with the CDC and with help of the LRN to inculcate the number of public health laboratories that can perform the test to over 67 laboratories across 48 states and the number of weekly tests were made available within the LRN to over 8,000 tests per week.
- With the approval from CDC LabCorp have access to increase capacity up to 10,000 tests per week, which will double the current capacity provided with the help of CDC’s Laboratory Response Network (LRN).
Regulatory Framework for Monkeypox Disease
"Protecting Public Health: Creating a Robust Regulatory Framework for Monkeypox Disease"
The regulatory framework for monkeypox infection is heavily facilitated by Disease Landscape Insights based on different countries’ regulatory norms. It offers data-driven insights that direct risk assessment, regulatory development, surveillance, import/export laws, research oversight, response to emergency planning, and collaboration across borders. Institutes and market players can effectively prevent, control, and respond to outbreaks of monkeypox while making sure that the affected communities are safe and well-cared for by integrating Disease Landscape into the regulatory framework.
Ongoing regulatory permissions and EUAs promote market expansion. For instance, the U.S. FDA authorized the intradermal injectable JYNNEOS vaccine for emergency use in August 2022 for medical personnel. Due to the similarities between smallpox and monkeypox, MVANEX has been recognized as an effective vaccine for monkeypox disease. The usage of the smallpox vaccine as an off-label product for vaccination against monkeypox and rising R&D spending are further factors that are projected to boost the global market. Among the variants in the vaccine, one of these (MVA-BN) and one (LC16) has received approval in Canada, Europe, and the United States of America, respectively. There are numerous antiviral drugs that can be used to treat monkeypox.
Competitive Analysis
"Unleashing Industry Opportunities: Explore the Monkeypox Market's Competitive Landscape"
- To satisfy the growing need for early detection and improve their competitive position, leading companies in the international market have been focusing on new product launches and expanding their testing capabilities In addition to that they are adopting organic and inorganic strategies for growth and expansion which majorly includes drug discovery regulatory approval and inorganic strategy such as acquisition partnership and majorly focusing on collaboration order to improve and grow their product portfolio.
- SIGA has established an end-to-end network with over 20 partners across drug discovery, pre-clinical, clinical, manufacturing, and supply chains that supported the development of TPOXX and the successful delivery of approximately US$ 200 million of courses to the Strategic National Stockpile
- SIGA pharmaceutical company concentrated on the health security market declared that they have entered a collaboration with oxford university in the UK to provide tecovirimat to treat people from suffering Monkeypox virus.
- In July 2022 Bavarian declared that they have additionally got 2.5 million of dosage jynoesee from USA Brada
- Chimerix Inc., an emerging biotechnology company developing orally available, targeted medicines, announced that it has licensed rights from Gilead Sciences, Incto develop oral derivatives of Gilead's proprietary antiviral compound cidofovir, for the prevention or treatment of smallpox infections and Monkeypox. Chimerix can now sell CMX001 to governments wherever in the globe because to the licence.
Market Trends Analysis
‘’Harness the changing patterns of the market in monkeypox to be ahead of the curve’’.
The market is expanding as a whole because of other technological developments and the use of PCR technology for testing. The NeuMoDx automated PCR method was developed by Qiagen in May 2022 to improve disease outbreak detection in non-endemic locations. This will facilitate more rapid diagnosis of monkeypox and better crisis management.
To promote patient outreach, monkeypox testing, and community education in US areas, GSK subsidiary VIIV will give USD 50,000 in August 2022. Along with that the global rise in vaccination demand and the prevalence of infection are projected to fuel market expansion.
The Department of Health and Human Services said in July 2022 that it would boost vaccine accessibility by acquiring an additional 144,000 doses of the JYNNEOS vaccine to treat monkeypox illness. The FDA is intended to encourage diagnosis and recommend EUA requests for monkeypox diagnostic tests under this strategy. Therefore, it is anticipated that such initiatives will advance the market.
Furthermore, the human medicines committee of the EMA suggested an expansion of the smallpox vaccine IMVANEX's indication to protect patients from monkeypox infection in July 2022. IMVANEX has been approved since 2013, but because smallpox and monkeypox are similar, it is thought to be a helpful vaccine against the monkeypox virus. Additionally, it is anticipated that growing R&D and the use of the smallpox vaccine as an off-label product for vaccination against monkeypox will propel the market globally. This indicates that the vaccination market will expand significantly and will create market growth opportunities for smallpox and monkeypox vaccine manufacturers.
Clinical assessment
Here the exciting journey into the world of monkeypox treatment gets underway in Disease Landscape, where novel advancements and exceptional revolutionary possibilities will transform the disease landscape.
Establishing and carrying out clinical trials for novel treatments and medications, patient recruitment strategies, regulatory compliance, ensuring positive trial outcomes, etc. are all made easier with the assistance of Disease Landscape. The study titles of the currently ongoing clinical trials, together with the phases in which they are currently being done, are provided in the table below.
|
PHASE 1 (Human Pharmacology) |
PHASE 2 (Therapeutic Exploratory Trail) |
PHASE 3 (Therapeutic Confirmatory Trial) |
PHASE 4 (Post Marketing Surveillance) |
|
|
A Randomized, Placebo-controlled, Double-blinded Trial of the Safety and Efficacy of Tecovirimat for the Treatment of Adult and Paediatric Patients with Monkeypox Virus Disease |
A Randomized, Placebo-Controlled, Double-Blinded Trial of the Safety and Efficacy of Tecovirimat for the Treatment of Human Monkeypox Virus Disease |
Cohort Events Monitoring (CEM) Study for the Assessment of Safety Profile of MVA-BN (Jynneos) Vaccine in Adult Personnel and Staff in the PALM-007 Study in Democratic Republic of The Congo |
|
|
A Phase 2 Randomized, Open-Label, Multisite Trial to Inform Public Health Strategies Involving the Use of MVA-BN Vaccine for Mpox |
A Phase III, Multi-country, Randomized, Placebo-controlled, Double-blinded Trial to Assess the Efficacy and Safety of Tecovirimat Antiviral Treatment for Patients with Monkeypox Virus Disease |
|
|
|
Monkeypox and Cancer: A Pan-cancer Based Multi-omics Analysis and Single Cell Sequencing Analysis-Experimental Studies |
Monkeypox and Cancer: A Pan-cancer Based Multi-omics Analysis and Single Cell Sequencing Analysis-Experimental Studies |
|
The monkeypox diseases market has some challenges and Disease Landscape Insights supports in providing solutions to the challenges faced by a market player in the monkeypox industry,
There are different market scenarios with respect to countries/regions for monkeypox-specific diagnostics and medications. Owing to the disease's modest impact & the challenges associated with commercial viability to treat diseases have generally shown different national investments in monkeypox-specific treatments.
Disease Landscape Insights aids in identifying the target market, driving strategic business plans, and keeping market participants informed of emerging trends. Disease Landscape insights services help manufacturers in developing and implementing effective medicines to halt and control monkeypox outbreaks.
In addition, there is greater demand for diagnostic devices, clinical assessment, and monkeypox vaccines because of expanding awareness and expected outbreaks.
Disease Landscape Insights here will provide crucial services across the business segment and the assistance provided at Disease Landscape Insights makes it simpler to establish and carry out clinical trials for novel therapies and pharmaceuticals, patient recruitment tactics, regulatory compliance, assuring favorable trial outcomes, etc.
This supports companies to perform focused research and development, learn about contract manufacturing organizations and CMOs, find raw material suppliers, and ensure legal compliance for the business all benefit from this.
Disease Landscape Insights (DLI) supports all market players in establishing their greater foothold in monkeypox disease industry.